Background and aims: Immunotherapy with anti-CD20-specific antibodies (e.g. rituximab), has become the standard of care for B cell lymphoproliferative disorders and many autoimmune diseases. Despite previously demonstrated role for B cells in bone metabolism, the effect of anti-CD20-mediated B cell depletion on bone mass in human patients has not been thoroughly studied. For example, in rheumatological patients the effect of rituximab on bone mass yielded conflicting results, while in lymphoma patients it has not yet been described. Here, we describe the effect of treatment with anti-CD20-specific antibodies on bone mass in a cohort of patients with follicular lymphoma and propose a plausible mechanism using murine model.
Methods: To assess the effect of rituximab on bone mass in lymphoma patients, we retrospectively studied the bone mass in patients with follicular lymphoma (FL) during the maintenance phase of chemoimunotherapy, i.e. when rituximab is administered as monotherapy. FL patients on no maintenance (historical controls) or patients with marginal zone lymphoma were include as a control group. Cross-sectional X-ray imaging (CT/PET-CT), performed at the completion of the induction phase and 6-12 months thereafter, were used to serially assess bone density. Wild-type female C57BL/6J mice and mouse anti-mouse CD20 antibody (Genentech) were used for the animal experimental system. Murine bone structure was assessed by the microCT method. Immunophenotyping of the bone marrow (BM), spleen and peripheral blood cells was performed. ELISA and "real-time" quantitative PCR were used to measure the levels of key mediators of bone remodeling, e.g. RANKL, OPG and TNFα. Standard osteoclastogenic assay was used to assesses the osteoclastogenic potential of BM cells.
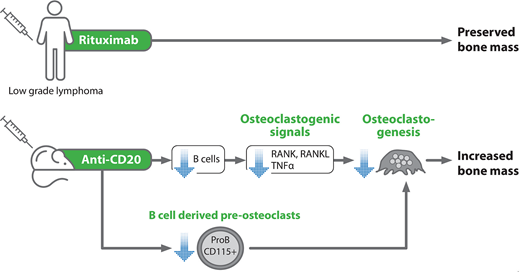
Results: Rituximab treatment prevented the decline in bone mass observed in patients who did not receive active maintenance therapy, both in the lumbar spine (-2.6% vs -8%) and femoral head (-0.5% vs -5.1%) (n=12 patients in each group, p<0.05 for the comparisons in the control group, calculated by Wilcoxon matched-pairs signed rank test). Anti-CD20-mediated B cell depletion in mice led to a significant increase in bone mass as reflected by: 7.7% increase in bone mineral density (whole femur) and ~5% increase in cortical as well as trabecular tissue mineral density (n=17-21 mice in each group, p<0.05). Mechanistically, treating mice with anti-CD20 antibodies significantly decreased the osteoclastogenic signals, including RANKL and TNFα, along with a substantial downregulation of RANK (the RANKL receptor). This correlated with nearly a 50% reduction in osteoclastogenic potential of BM cells derived from B-cell-depleted animals (p<0.05). The decline in the RANKL output was observed both at the bone level (≈25% relative reduction in the mRNA levels), as measured in the whole bone and BM cells, as well as in the serum (18% relative reduction) of the anti-CD20-treated mice as compared to diluent-treated controls (p<0.05 for all comparisons). No significant changes in the OPG levels were noted.
Conclusions: While many lymphoma patients may suffer from bone loss due to advanced age and glucocorticoid administration, our data suggest additional favorable effect of anti-CD20 treatment in bone preservation. Importantly, our murine studies indicate that B cell depletion has a direct effect on bone remodeling, primarily by reducing the osteoclastogenic signals, thus potentially diminishing bone resorption. This novel unrecognized effect should be taken into consideration when maintenance treatment is considered.
MM and DN contributed equally to this work.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal